About G.T.J. (Bert) van der Horst, PhD
Introduction

Bert van der Horst studied biology at the University of Amsterdam. In 1993, he received his PhD in Cell Biology at the Erasmus University Rotterdam, where he studied the lysosomal enzyme sialidase. As a postdoctoral fellow at the Department of Molecular Genetics, Erasmus MC, he has generated and characterized a large panel of (conditional) animal models for DNA repair disorders, associated with skin cancer predisposition and/or premature aging (e.g. Cockayne syndrome), as well as for mammalian homologs of the repair protein photolyase (mCry1 and mCry2). Unexpectedly, the mCry genes were shown to encode indispensable components of the biological clock, a major breakthrough in the field of chronobiology. For the latter work he received a prestigious ZonMW Vici award that stood at the basis of a new research line in the Department, focusing on the molecular mechanism and biological impact of the circadian system.
By integrating running research lines on the circadian clock, genome instability and aging in a new multidisciplinary research direction, and by applying state of the art technology (i.e. conditional mouse models, clock-synchronized cultured cells, transcriptomics, miRNA profiling, in vivo imaging in cells, tissues and animals), the clock team aims at obtaining fundamental knowledge on the mechanism and biological/medical impact of the circadian system, with emphasis on the (bidirectional) relation between the circadian system on the one hand, and the origin of cancer and the aetiology of age-related disorders on the other hand, including the adverse effect of chronic circadian disturbance (i.e. jetlag, shift work) thereon. Recently, the group became interested in how pre- and postnatal environmental clock-disturbing conditions may (epigenetically) influence the programming of the circadian system and how this may impact on later life health (DOHaD: Developmental Origin of Health and Disease). Ultimate goal is to translate this knowledge into applications to prevent or delay onset of aforementioned disorders (preventive intervention) and into new clinical approaches in cancer and other drug-based therapies (personalized medicine, chronotherapy).
Running research lines
The circadian clock and cancer
Circadian misalignment and health
Field(s) of expertise
General Introduction
The circadian clock is an internal timekeeping system that imposes 24 hour periodicity on behavior, physiology and metabolism and allows us to anticipate the momentum of the day. Using living organisms (mice, humans) and (single) cell culture methods, we study how genetic disruption (i.e. clock gene mutations) or environmental disturbance (i.e. shift work, inherent to our 24/7 society) of the circadian clock may contribute to the onset of disease. Conversely, we investigate how we can make use of the circadian clock to optimize therapeutic efficacy and minimize side effects of pharmaceutical drugs.

Introduction to the circadian system
Like most organisms, we have developed an internal time keeping system that drives daily rhythms in metabolism, physiology and behavior, and allows us to optimally anticipate to the momentum of the day. At the basis of circadian timekeeping lies an intracellular molecular oscillator in which a set of clock genes cyclically regulate their own expression with an approximate (circa) 24-hour (dies) periodicity. The mammalian circadian system consists of a light-entrainable master clock in the neurons of the suprachiasmatic nucleus (SCN) in the brain, and light-irresponsive peripheral clocks in the cells of virtually all other tissues. As the circadian clock drives rhythmic expression of up to 10 % of the active genes (thereby conferring rhythmicity to a wide range of cellular processes such as, but certainly not limited to, energy metabolism, metabolic activation of drugs, detoxification, hormone synthesis, DNA repair and cell cycle control), it may not come as a surprise that disruption of the circadian system is associated with disease. Indeed, genetic disruption of the circadian system in rodent models by inactivation of clock genes has been found to increase tumor growth, accelerate aging, and disrupt metabolism. On the other hand, our 24/7 economy requires many people to work at “non-standard” times. Recently, epidemiological and experimental animal studies have revealed a relation between disturbance of our body clock by repeated shift-work and an increased risk for developing pathologies such as cancer, metabolic syndrome and cardiovascular disease.
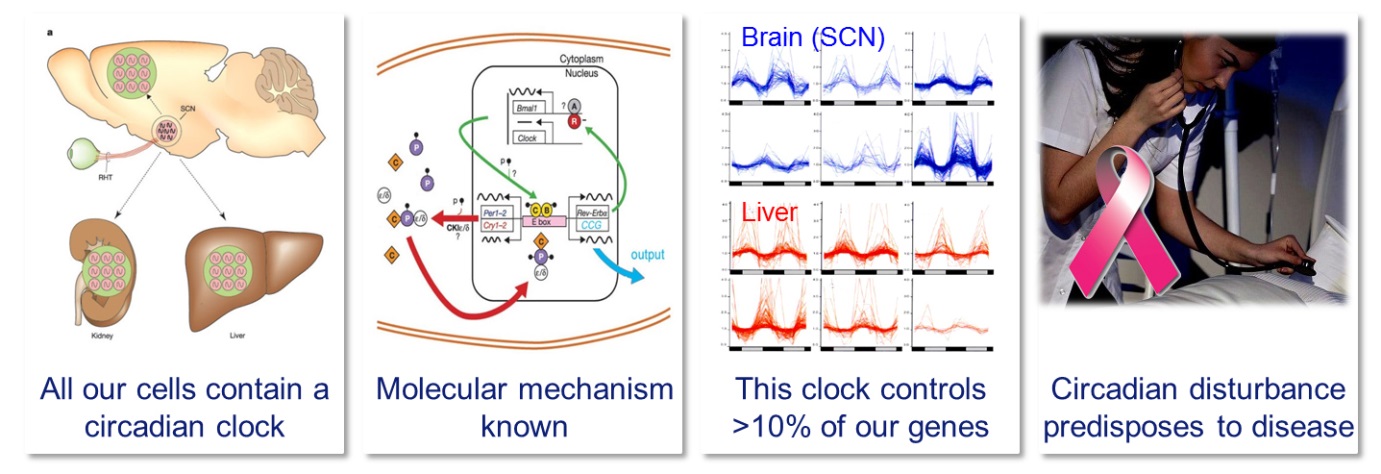
Four important facts about the circadian clock
The circadian clock and cancer therapy
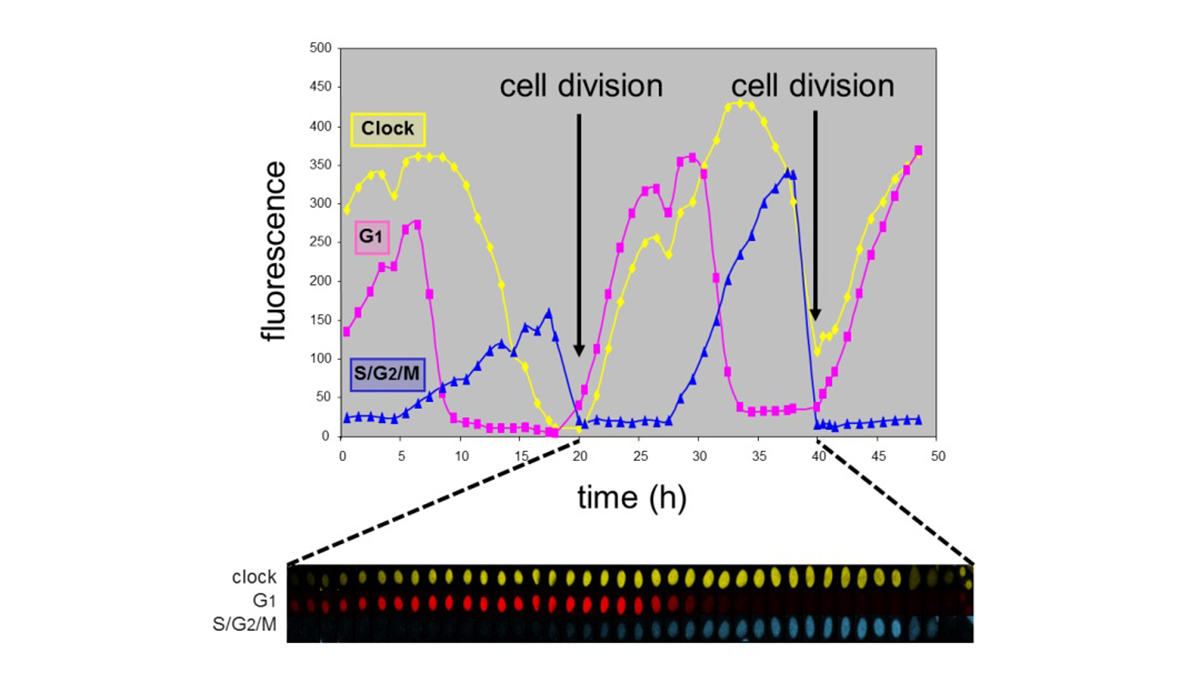
In healthy cells the circadian clock and cell cycle are phase-coupled
Evidently, fundamental research on the link between the circadian system and the cell cycle, genome maintenance, tumor induction and tumor progression, and subsequent implementation of this knowledge in the development of intervention and therapeutic strategies will advance our combat against cancer. As part of our efforts to replace animal studies for risk assessment, we are establishing in vitro assays for determining the chronotoxic properties of (geno)toxic agents in relation to time of the day of exposure. Moreover, chronotoxic properties of compounds can be very useful when treating cancer with chemotherapeutic or other anti-cancer agents. Chronomodulated therapy (chronotherapy) involves protocols in which chemotherapeutic agents are given at a specific moment of the day, notably at the time when healthy cells are least sensitive. Although chronotherapy is a recognized approach, it is not generally applied. This represents a huge waste of resources and clinical opportunities for the patient.

Circadian misalignment and health
A major societal concern of today’s 24/7 economy is to what extent (chronic) disturbance of our regular biological rhythm (circadian misalignment), as is the case with travelers adapting to a new time zone and laborers working at non-office hours, contributes to the development of serious adverse later-life health effects. Epidemiological studies have associated frequent jet lag and shift work with an increased incidence of metabolic disorders, obesity, cardiovascular disease and cancer. Moreover, using an animal experimental approach, we recently provided the first experimental proof that chronic circadian rhythm disturbance is a breast cancer risk factor [Van Dycke et al., 2015].

Chronic misalignment between our body time and mechanical time is a health risk factor
Social jet lag as a health risk factor An often underestimated form of chronic circadian rhythm disturbance is “social jet lag”, which is defined as a discrepancy between social timing (imposed by our society) and biological timing (dictated by our internal body clock). Social jet lag is the result of reduced sleeping times on working days (>2 hours/night for 30% of the population!), as compared to free days. Only recently, epidemiological studies have associated social jet lag with an increased risk to develop obesity, metabolic disorder and diabetes type 2. In collaboration with the he National Institute for Public Health and the Environment RIVM, Bilthoven; Dr. Linda van Kerkhof), we are currently investigating to what extent chronic social jet lag is a cancer and/or other health risk factor.
Pre- and postnatal circadian disturbance and later life health We recently obtained evidence that, in line with the Developmental Origin of Health and Disease (DOHaD) hypothesis, exposure of pregnant mice to chronic jetlag or continuous exposure of mice to light in the first 4 weeks after birth, affects circadian development of the offspring and predisposes to health effects in adult life. In collaboration with the Departments of Neonatology (Prof. dr. Irwin Reiss) and Epidemiology (Prof.dr. Vincent Jaddoe), we are currently following a multidisciplinary approach involving animal and human studies (Generation R) to test the hypothesis that aberrant pre- and postnatal light conditions can leave epigenetic marks on the newborn genome that remain present throughout life and that touch upon circadian performance and later life health.

Mouse studies suggest that pre- and early postnatal circadian disturbance affects later life health of the child and accordingly is a DOHaD risk factor
Biomarkers for long term circadian disturbance Since demanding work schedules are a fact of life in modern society, efforts are needed to better understand how shift work affects the body and to develop shift work schedules with minimal adverse health effects. This will not only be beneficial for public health, but also contribute to controlling health care costs. We therefore aim at developing biomarkers that allow a quantification/assessment of chronic circadian disturbance and develop evidence-based measures to sustain fitness and employability of shift workers. As part of these efforts, we are currently investigating how the human body responds to prolonged exposure to extreme physical and circadian stress, encountered during the heavily demanding Volvo Ocean Race in which sailors perform in 4 hour on, 4 hour off watches. What are the short and long term health effects of chronic stress exposure and are these effects reversible? We expect that the knowledge obtained will help to develop strategies that enable the body to better anticipate external stressful environmental factors associated with shift work.
Education and career
Positions
- 2007-present Professor Chronobiology & Health”, Dept. of Molecular Genetics (Prof.dr. R. Kanaar), Erasmus University Medical Center, Rotterdam, NL
- 2001-2007 Associate professor, Dept. of Genetics (Prof.dr. J.H.J. Hoeijmakers), Erasmus University Medical Center, Rotterdam, NL
- 1997-2001 Assistant professor, Dept. of Cell Biology and Genetics, Inst. of Genetics (Prof.dr. D. Bootsma, Prof.dr. J.H.J. Hoeijmakers), Erasmus University Rotterdam, NL
- 1993-1997 Research associate, Dept. of Cell Biology and Genetics, Inst. of Genetics (Prof.dr. D. Bootsma, Prof.dr. J.H.J. Hoeijmakers), Erasmus University Rotterdam, NL
- 1986-1993 Research associate, Dept. of Cell Biology and Genetics, Inst. of Cell Biology (Prof.dr. H. Galjaard), Erasmus University Rotterdam, NL
- 1983-1985 Research associate, Lab. of Biochemistry, section Molecular Biology (Prof.dr. L.A. Grivell), University of Amsterdam, NL






